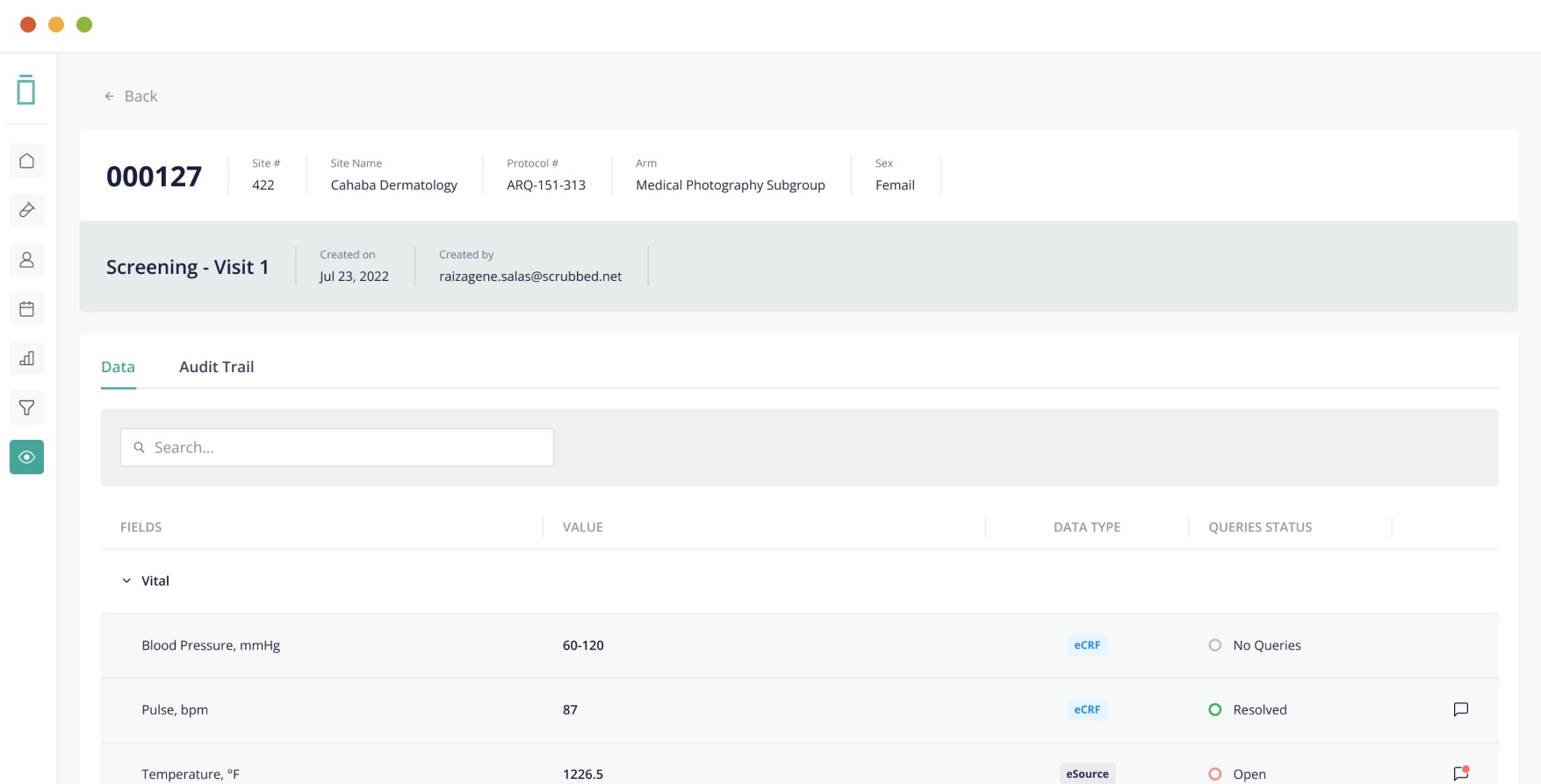
Electronic Data Capture in Clinical Trials
If you’re unfamiliar with electronic data capture, this article will explain the basics, including what it is, how it works, and the benefits of using it. We’ll also discuss the costs and the process of implementing an electronic data capture system in a clinical trial. Let’s get start.
Electron capture
Electronic capture is a great way to streamline the collection and usage of data. It helps keep records more secure, accessible, and searchable. It makes data collection faster and more accurate. In the world of financial services, speed and efficiency are highly valued. Luckily, electronic capture technology is easy to use and offers many advantages.
Electronic data capture (EDC) systems allow research institutions to collect data in a more efficient manner than ever before. These systems use electronic data capture tools to digitize clinical trial data. Many of these tools are cloud-base, meaning that researchers can access them from anywhere. They can also be use to streamline the collection and management of patient data and other information.
EDC systems are important to increasing the speed and convenience of customer experiences. EDC systems are design to prevent errors during data capture. Additionally, they are design to ensure that all data is correct and up-to-date.
Many companies use EDC methods for their clinical trials
Benefits include increase efficiency, cost reduction, data security, and accessibility. These benefits make EDC systems an important technology for businesses. Electronic data capture systems help businesses capture data without having to worry about manual data entry.
Electronic data capture systems help researchers collect and organize their study data in a consistent manner. They help reduce data entry errors, improve data quality, and speed up the approval process for clinical trials. In addition, these systems also support the creation of documentation for researchers. This means that researchers can save time and money while performing their research.
Many healthcare organizations use EDC systems to capture specific data about patients and individuals. For example, a bio-pharmaceutical company may test a new diabetes drug on 200 people in 10 medical centers. Each medical center enters the study data using an EDC system. These systems are also useful for research and development and are often use in pharmacological studies.
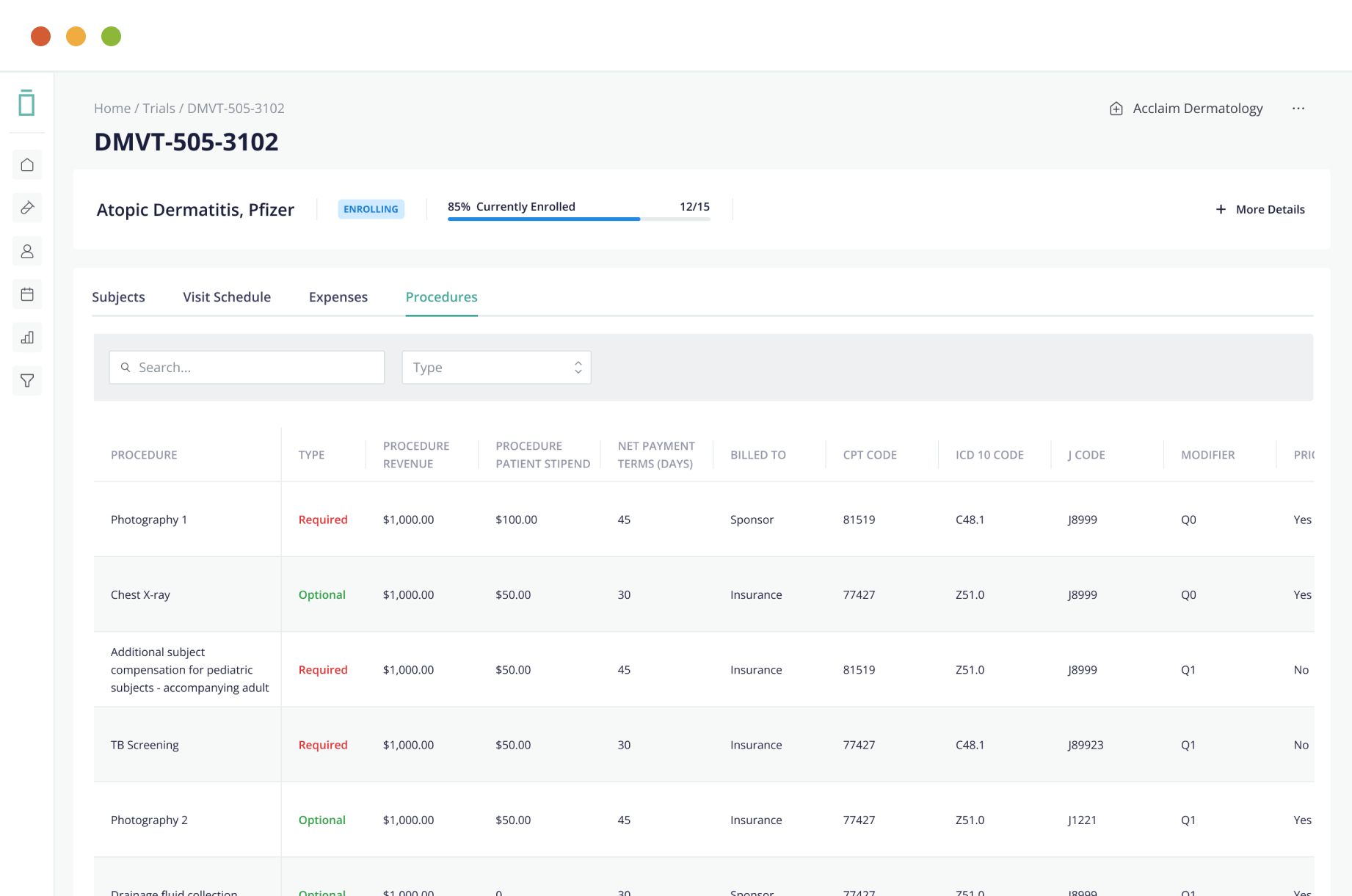
Benefits of electronic data capture
Electronic Data Capture is an important process that helps companies automate their document processing. It can be used to process invoices, orders, bank statements, patient records, and more. Also it can automate data entry and sorting, which will save both time and resources. In addition, it can store information in an online document management system for increased visibility and a complete audit trail. With this method, businesses can eliminate manual data entry and can focus on getting the best results for their customers.
Electronic Data Capture (EDC) has evolve from a time of human error and inefficient IT systems. It has become popular in the past decade, especially in clinical trials. With the increased volume of data, the medical industry has embrace the use of new technologies and electronic record keeping. This technology makes it possible to capture and analyze large amounts of data in a timely manner.
Data capture can also help streamline research workflow
Researchers only have to input data once, reducing the number of staff members and resources needed to complete their work. This technology also reduces error rates, which saves valuable time. For example, an electronic data capture system like ClinCapture can catch mistakes as they occur. This means that the researcher does not have to go through the tedious task of correcting mistakes manually.
Electronic Data Capture systems can be hosted on premise, in the public cloud, or in a private clinical cloud. Public cloud software is generally cheaper, but it may require frequent training and re-validation. Private cloud software is ideal for organizations that want the benefits of being “on the cloud” without the disruptions.
In clinical trials, EDC systems help researchers collect data faster and more efficiently. Moreover, they help in maintaining data integrity. EDC can eliminate preventable errors and fraudulent data entry. Further, electronic data capture makes it possible to export data in FDA-approved formats. If this technology is used, it can make the research process much faster and cheaper.
EDC systems also help researchers manage data quality. Researchers can add dependencies and validate forms electronically, which will increase data quality. The system will also flag errors and results that fall outside of parameters. These features help researchers minimize the risk of human error and improve the quality of data.
Read also: What’s the Difference Between a Neurologist and a Neurosurgeon?
Costs
Automatic electronic data capture (EDC) is the latest trend in the financial sector. It allows employees to focus on high-level tasks rather than processing countless paper documents. EDC can be configured to occur at any stage of the document’s lifecycle. It eliminates the costs associated with handling physical paper, such as courier services and storage.
While the technology has not been demonstrated at a large scale, cost estimates vary widely. Some companies estimate that costs can run between USD 100/t and $1000/t. Costs vary based on energy costs, financial assumptions, and plant configuration. Currently, there are 19 DAC plants operating in the United States and Europe. Most of them are small and sell captured CO2 for use in carbonating beverages.
Although a capture system can be expensive to purchase
It is a worthwhile investment if it enhances the security of patient data and decreases the response time to patient queries. Despite the upfront costs, the advantages of document management are great and can help businesses succeed in the healthcare industry.
Further, MACRO allows for an online and offline data entry facility, which can help reduce the cost of the system. In addition, CICS offers the option to implement individual trials. However, additional licensing costs may be required for named users. These costs must be covered by the sponsor.
In addition to costs, an electronic capture system may also involve training staff members. Some systems can capture all types of documents and tag metadata to improve security. Some of these systems also allow for integration with third-party consultants and internal resources. These services can include software releases, audits, escrow, and training for systems administrators, scanning agents, and end users.
An EDC system can also help businesses reduce costs related to manual data capture mistakes. It helps to ensure data integrity by allowing real-time error checking. EDC can also be more time efficient to capture data. EDC reduces the need for manual data entry, which can lead to a significant reduction in costs.
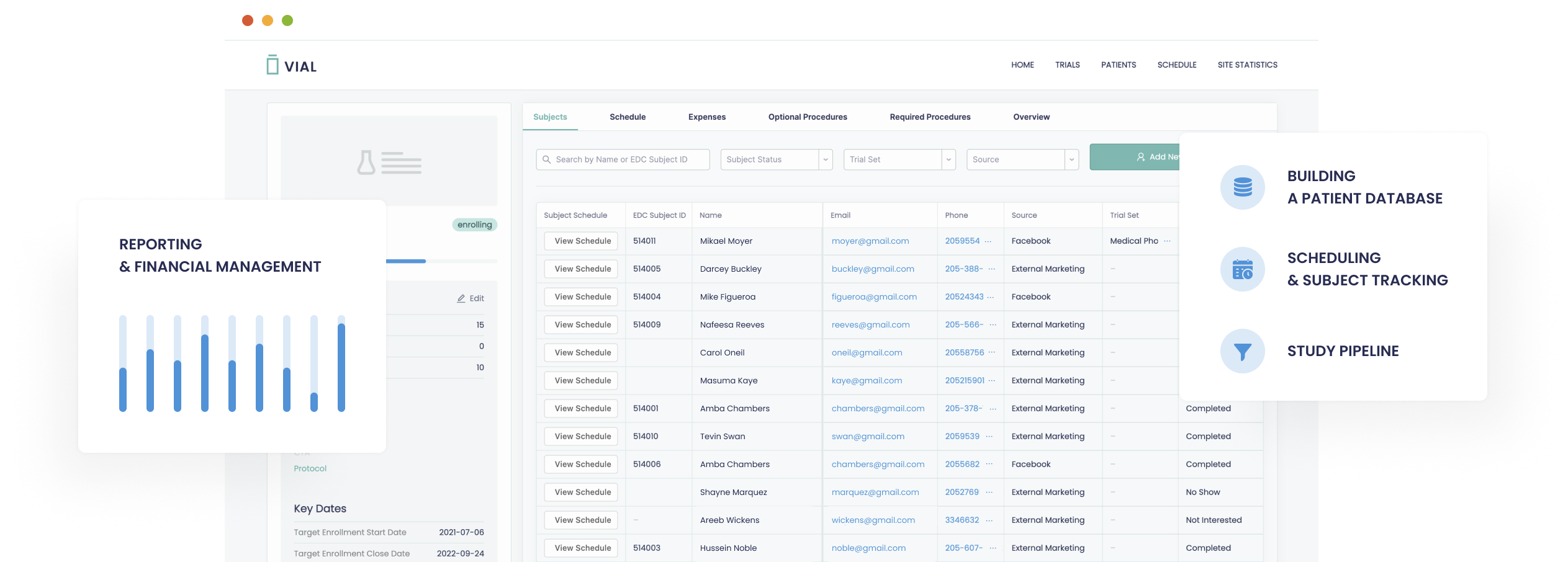
Process of implementing an electronic data capture system in a clinical trial
EDC can help increase efficiency and accuracy in running clinical trials. But before you implement it, you need to ensure that it will work as planned. To avoid errors, ensure that the system has features that ensure it is secure and compliant. There are several ways to do that.
Electronic data capture systems are computer programs that help researchers enter and manage data from clinical trials. These programs provide a convenient method for clinical trial sites to collect and store data, including patient visits. An effective electronic data capture system can reduce human error, speed up the trial, and reduce research costs. Many healthcare organizations are implementing custom software development projects to capture clinical data.
EDC software can be purchased or built on-site
These systems allow research teams to collect data faster than they can with paper CRFs. EDC software can also save time during data refinement and cleaning, and help to create a database structure in a fraction of the time.
Electronic data capture systems also enable researchers to add dependencies, validate forms, and reduce human error. It ensures the accuracy of data collected and flags data that are not within the parameters set. This helps reduce human error and improve the quality of data. And it reduces costs of on-site monitoring and administrative staff.
The EDC system must also be configure to incorporate a reporting functionality. A good EDC system must provide a basic set of reports that clinical site staff can use to monitor their activity. Ideally, these reports can also include action item tracking and adverse event reports.